HCV Testing and Linkage to Care
One-Time Hepatitis C Testing
Recommendations for One-Time Hepatitis C Testing |
|
|---|---|
| RECOMMENDED |
RATING |
| One-time, routine, opt out HCV testing is recommended for all individuals aged 18 years or older. | I, B |
| One-time HCV testing should be performed for all persons less than 18 years old with activities, exposures, or conditions or circumstances associated with an increased risk of HCV infection (see below). | I, B |
| Prenatal HCV testing as part of routine prenatal care is recommended with each pregnancy. | I, B |
| Periodic repeat HCV testing should be offered to all persons with activities, exposures, or conditions or circumstances associated with an increased risk of HCV exposure (see below). | IIa, C |
| Annual HCV testing is recommended for all persons who inject drugs, for HIV-infected men who have unprotected sex with men, and men who have sex with men taking pre-exposure prophylaxis (PrEP). | IIa, C |
|
Risk Activities
Risk Exposures
Other Conditions and Circumstances
|
|
Based on the 2013–2016 National Health and Nutrition Examination Survey (NHANES) data among the general noninstitutionalized US population, an estimated 4.1 million people have had HCV exposure (HCV-antibody–positive), including 2.4 million with active HCV infection (HCV-RNA–positive) (Hofmeister, 2019). Total HCV burden in the US also includes those not accounted for in NHANES data—incarcerated, institutionalized, or unsheltered homeless persons—with estimates ranging from 380,000 to 800,000 additional HCV-antibody–positive persons (Hofmeister, 2019); (Edlin, 2015). Approximately 50% of all infected persons are unaware that they have HCV (Yehia, 2014); (Holmberg, 2013); (Denniston, 2012).
HCV screening is recommended because of the known benefits of care and treatment in reducing the risk of decompensated cirrhosis, hepatocellular carcinoma, and all-cause mortality, and the potential public health benefit of reducing transmission through early treatment, viral clearance, and reduced risk behaviors (Chou, 2020); (Owens, 2020); (Schillie, 2020); (Smith, 2012).
HCV is primarily transmitted through percutaneous exposure to infected blood. Other modes of transmission include mother-to-infant and contaminated devices shared for noninjection drug use. Sexual transmission also occurs but is generally inefficient except among HIV-infected men who have unprotected sex with men (Pakianathan, 2018).
Injection drug use (IDU) poses the greatest risk for HCV infection, accounting for at least 60% of acute HCV infections in the United States. Healthcare exposures are important sources of transmission, including: the receipt of blood products in the US prior to 1992 (after which routine screening of the blood supply was implemented); receipt of clotting factor concentrates in the US before 1987; receipt of blood or blood products in other countries (risk depends on country prevalence and screening practices); long-term hemodialysis; needlestick injuries among healthcare workers; and patient-to-patient transmission resulting from poor infection control practices. Other risk factors include having been born to an HCV-infected mother, incarceration, and percutaneous or parenteral exposures in an unregulated setting. Examples include tattoos received outside of licensed parlors and medical procedures performed internationally or domestically where strict infection control procedures may not have been followed (eg, surgery before implementation of universal precautions) (Hellard, 2004).
The importance of these risk factors might differ based on geographic location and population (Schillie, 2020); (Owens, 2020). An estimated 12% to 39% of incarcerated persons in North America are HCV-antibody–positive, supporting the recommendation to test this population for HCV infection (Larney, 2013); (Allen, 2003); (Weinbaum, 2003).
Because of shared transmission modes, persons with HIV infection are at risk for HCV. Annual HCV testing is recommended for sexually active HIV-infected adolescent and adult men who have sex with men. The presence of concomitant ulcerative sexually transmitted infections, proctitis related to sexually transmitted infections, or high-risk sexual or drug use practices may warrant more frequent testing. Sexual transmission is particularly a risk for HIV-infected men who have unprotected sex with men (Hosein, 2013); (van de Laar, 2010). Testing sexually active, non-HIV–infected persons for HCV and HBV infection before starting and while receiving pre-exposure prophylaxis (PrEP) for HIV prevention should also be considered (Hoornenborg, 2020); (Volk, 2015).
Data also support testing in all deceased and living solid organ donors and all recipients because of the risk of HCV infection posed to the recipient (Lai, 2013); (Jones, 2020). Although hepatitis C testing guidelines from the US Centers for Disease Control and Prevention (CDC) and the US Preventive Services Task Force (USPSTF) do not specifically recommend testing immigrants from countries with a high HCV prevalence (eg, Egypt and Pakistan), such persons 18 years or older are included in the one-time, opt out HCV testing recommendation.
CDC established risk-based HCV testing guidelines in 1998 (CDC, 1998). These guidelines were expanded in 2012 with a recommendation to offer one-time HCV testing to all persons born from 1945 through 1965 without prior ascertainment of HCV risk factors. This recommendation was supported by evidence demonstrating that persons in this age group had a 6-fold higher prevalence of HCV infection and that a risk-based strategy alone failed to identify >50% of HCV infections, due in part to patient underreporting of their risk and provider limitations in ascertaining risk factor information (Denniston, 2012). The USPSTF also recommended one-time HCV testing in asymptomatic persons belonging to the 1945 through 1965 birth cohort as well as other individuals based on exposures, behaviors, and conditions or circumstances that increase HCV infection risk.
Since the birth cohort recommendation was adopted, however, there has been an increase in the number of acute and chronic HCV infections reported in individuals born after 1965 (Zibbell, 2018); (Ly, 2017); (Suryaprasad, 2014). The increase in HCV incidence and prevalence among a younger cohort results from the opioid epidemic and increased IDU. This shift in HCV epidemiology and the known failures of risk-based testing warranted an expansion of the recommendation. Accordingly, CDC updated screening recommendations in 2020 to include HCV screening at least once in a lifetime for all adults aged ≥18 years as well as HCV screening of all pregnant women during each pregnancy, except in settings where the prevalence of HCV infection (ie, HCV-RNA positivity) is <0.1%. Both recommendations were based on extensive literature review and estimates of the cost-effectiveness of screening. In their recommendation, CDC noted that no states have an estimated HCV-RNA prevalence <0.1% (Schillie, 2020). USPSTF also recently issued recommendations for one-time, routine, opt-out testing of adults aged 18 through 79 years (Owens, 2020).
For the CDC 2020 testing guidelines, a systematic review included a harm assessment for HCV screening during pregnancy. This review was augmented by input from subject matter experts, studies not captured through the formal literature review, and the peer-review process. Despite several plausible harms (including insurability and employability issues, legal ramifications and potential loss of infant custody, unnecessary cesarean deliveries, and unnecessary avoidance of breastfeeding), CDC concluded that identified or potential harms did not outweigh the benefits of HCV screening (Schillie, 2020).
Generally, routine HCV testing is cost-effective because of increasing HCV incidence and prevalence among people who inject drugs (PWID) and the decreasing cost of DAA therapy. Many patients at greatest risk for HCV infection and transmission do not readily report their highly stigmatized risk activities. Studies conducted in US urban emergency departments, for example, reveal that 15% to 25% of patients with previously unidentified HCV infection were born after 1965 and/or have no reported history of IDU and are, therefore, missed by even perfect implementation of risk-based testing guidance (Schechter-Perkins, 2018); (Hsieh, 2016); (Lyons, 2016). Reinfection among those actively using drugs is common, but because HCV testing is a low-cost intervention and therapy is both highly effective and cost-effective, routine testing provides good economic value (ie, cost-effectiveness) even when many people need to be tested and treated more than once during their lifetime.
Several cost-effectiveness studies published since release of the birth cohort recommendations have demonstrated that routine, one-time HCV testing among all adults in the US would likely identify a substantial number of HCV cases that would otherwise be missed, and that doing so would be cost-effective. One research group employed simulation modeling to compare several versions of routine guidance, including routine testing for adults aged ≥40 years, ≥30 years, and ≥18 years. The investigators found that routine HCV testing for all adults ≥18 years was cost-effective compared to risk-based screening guidance, and potentially cost-saving compared to testing only those aged ≥30 years or ≥40 years (Barocas, 2018). The study further demonstrated that routine testing remained cost-effective unless HCV infection had no impact on healthcare utilization and no impact on quality of life. Another research team similarly found that routine HCV testing for all adults aged ≥18 years is likely cost-effective compared to risk-based screening guidance, provided the HCV prevalence among those born after 1965 is >0.07% (Eckman, 2019). Notably, these studies reached similar conclusions despite being conducted independently and employing different simulation modeling approaches. Further, a variety of studies have tested the cost-effectiveness of routine HCV testing in specific venues, including correctional settings (He, 2016), substance use treatment centers (Schackman, 2018); (Schackman, 2015), and federally qualified health centers (Assoumou, 2018). All of these studies demonstrated that routine HCV testing and treatment was cost-effective, even when linkage to HCV treatment after testing was poor and the rate of HCV reinfection among injection drug users was high.
Analyses focusing on pregnant women have demonstrated similar findings. One analysis calculated an incremental cost-effectiveness ratio (ICER) of $2,826 per quality-adjusted life-year (QALY) gained for universal screening of pregnant women compared with risk-based screening at an HCV-RNA positivity prevalence of 0.73% (Chaillon, 2019). Although real-world data informing screening during each pregnancy are lacking, a modeled analysis suggested that hepatitis C screening during each pregnancy would be cost-effective. Using a hepatitis C prevalence of 0.38% among pregnant women, the analysis found that universal hepatitis C screening during the first trimester of each pregnancy compared with the practice of risk-based screening had an ICER of $41,000 per QALY gained (Tasillo, 2019). Universal screening reduced HCV-attributable mortality by 16% and increased the proportion of infants identified as HCV-exposed from 44% to 92%. ICER remained ≤ $100,000 per QALY gained if hepatitis C prevalence was higher than 0.16% (Schillie, 2020).
Evidence regarding the frequency of HCV testing in persons at risk for ongoing exposures to the virus is lacking. Clinicians should, therefore, determine the periodicity of testing based on the risk of infection or reinfection. Because of the high incidence of HCV infection among PWID and HIV-infected men who have unprotected sex with men, HCV testing at least annually using an assay that detects HCV RNA (ie, a quantitative HCV-RNA test) if they have been previously exposed, is recommended among such individuals (Newsum, 2017); (Aberg, 2014); (Witt, 2013); (Bravo, 2012); (Linas, 2012); (Wandeler, 2012); (Williams, 2011).
Implementation of clinical decision support tools or prompts for HCV testing in electronic health records could facilitate reminding clinicians of HCV testing when indicated (Hsu, 2013); (Litwin, 2012).
Initial HCV Testing and Follow-Up
All persons for whom HCV screening is recommended should initially be tested for HCV antibody (CDC, 2013); (Alter, 2003) using an assay approved by the US Food and Drug Administration (FDA). A list of current FDA-approved HCV screening assays can be found on the agency website. FDA-approved tests include laboratory-based assays and a point-of-care assay (ie, OraQuick™ HCV rapid antibody test [OraSure Technologies]) (Lee, 2011). The latter is an indirect immunoassay with a sensitivity and specificity similar to those of laboratory-based HCV-antibody assays. Point-of-care assays are valuable in the community setting and allow for sample collection with a finger stick rather than standard phlebotomy. If point-of-care assays are used, reporting of results to the medical record and health authorities should follow protocols used for laboratory-based HCV-antibody tests. When possible, positive point-of-care antibody tests should be followed-up with immediate HCV-RNA confirmatory testing rather than referring the patient to another provider or setting to have the test performed. A study evaluating the performance parameters of the OraQuick™ HCV rapid antibody point-of-care test showed that people with viremia have higher antibody levels (compared with nonviremic persons), leading to a more rapid positive test result. All 227 viremic individuals in the study (from both clinical and real-world testing cohorts) tested positive within 5 minutes (Smookler, 2020). Based on a sensitivity of 100% (95% CI, 98.4-100%) in this study, if the OraQuick™ HCV rapid antibody test is not showing a positive result by 5 minutes, it is highly unlikely the person has active infection. Additional validation would be valuable, however, utilizing this so-called 5-minute rule may be considered, particularly in populations unable to wait the recommended 20–40 minutes before reading the test, or in high-throughput testing scenarios.
A positive HCV-antibody test indicates current (active) HCV infection (acute or chronic); past infection that has resolved; or a rare false positive (Pawlotsky, 2002). A test to detect HCV viremia is therefore necessary to confirm active HCV infection and guide clinical management, including initiation of HCV treatment. Many reference laboratories offer HCV-antibody testing that automatically reflexes to HCV-RNA PCR testing if the antibody test is positive. This should be considered the optimal testing approach in a clinical setting because it requires only a single blood draw without the need to bring people back to care for confirmatory testing, a major barrier in the continuum of care (Mera, 2016). HCV RNA point-of-care tests are also under evaluation (eg, Xpert® HCV viral load and Genedrive® HCV ID), which would allow for a rapid confirmation of viremia and immediate/same day treatment initiation. Point-of-care HCV-RNA tests are not yet FDA approved, as of this writing. Collection of dried blood spot (DBS) samples also allows for assessment of HCV antibodies and reflex HCV-RNA testing by testing spots sequentially. DBS samples can be collected using a finger stick rather than phlebotomy and can be transported without an intact cold chain, making it useful in rural areas and in people for whom phlebotomy may be a testing barrier (Lange, 2017).
HCV-RNA testing should also be performed in persons with a negative HCV-antibody test who are either immunocompromised (eg, persons receiving chronic hemodialysis) (KDIGO, 2008) or might have been exposed to HCV within the last 6 months because these persons may be HCV-antibody negative. An HCV-RNA test is also needed to detect reinfection in HCV-antibody–positive persons after previous spontaneous or treatment-related viral clearance.
Detection of HCV core antigen in the blood also indicates active HCV infection. Because the sensitivity of HCV core antigen testing is less than that of HCV-RNA testing, if an HCV core antigen test is used to assess viremia, antibody-positive samples that test negative for HCV core antigen should have a confirmatory HCV-RNA test to exclude a false negative core antigen result (van Tilborg, 2018).
An FDA-approved quantitative or qualitative HCV-RNA test with a detection level of ≤25 IU/mL should be used to detect HCV RNA. Figure 1 shows the CDC-recommended HCV testing algorithm.
Figure 1. CDC-Recommended Testing Sequence for Identifying Current HCV Infection
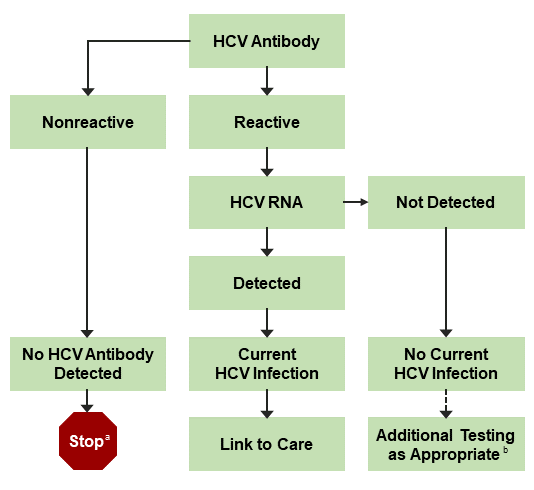
a For persons who might have been exposed to HCV within the past 6 months, testing for HCV RNA or follow-up testing for HCV antibody should be performed. For persons who are immunocompromised, testing for HCV RNA should be performed.
b To differentiate past, resolved HCV infection from biologic false positivity for HCV antibody, testing with another HCV-antibody assay can be considered. Repeat HCV-RNA testing if the person tested is suspected to have had HCV exposure within the past 6 months or has clinical evidence of HCV disease, or if there is concern regarding the handling or storage of the test specimen.
Adapted from Centers for Disease Control and Prevention (CDC, 2013).
Persons who have a positive HCV-antibody test and negative results for HCV RNA by PCR should be informed that they do not have laboratory evidence of current HCV infection, although it is possible that they may have had a previous exposure. Additional HCV testing is typically unnecessary. The HCV-RNA test can be repeated when there is a high index of suspicion for recent infection or in patients with ongoing HCV infection risk. They should also be informed that despite the presence of antibodies, they are not protected from infection/reinfection.
Clinicians (or patients) may seek additional testing to determine whether a positive HCV-antibody test represents a remote, resolved HCV infection or a false positive. For patients with no apparent risk for HCV infection, the likelihood of a false positive HCV-antibody test is related to the HCV prevalence in the tested population. False positive HCV-antibody tests most commonly occur in populations with a low prevalence of HCV infection (Alter, 2003). If further testing is desired to distinguish between a true positive vs biologic false positivity for HCV antibody, repeat testing may be performed using a different FDA-approved, HCV-antibody assay. A biologic false result should not occur with 2 different assays because they target different regions of the virus, making it highly unlikely that both would falsely detect a cross-reactive antigen (CDC, 2013); (Vermeersch, 2008).
Prior to initiation of antiviral therapy, quantitative HCV-RNA testing should be used to determine the baseline level of viremia (ie, viral load), which may affect treatment duration with certain regimens. The degree of viral load decline after initiation of treatment is less predictive of sustained virologic response (SVR) in the era of direct-acting antiviral (DAA) therapy compared with previous interferon-based treatment (see Pretreatment and On-Treatment Monitoring).
With the advent of pangenotypic HCV treatment regimens, HCV genotyping is no longer required prior to treatment initiation for all individuals. In those with evidence of cirrhosis and/or past unsuccessful HCV treatment, treatment regimens may differ by genotype and thus pretreatment genotyping is recommended (see Treatment-Naive and Treatment-Experienced sections). For noncirrhotic treatment-naive patients, although genotyping may impact the preferred treatment approach, it is not required if a pangenotypic regimen is used (see Simplified Treatment Algorithm).
Counseling Persons With Active HCV Infection
Recommendations for Counseling Persons With Active HCV Infection |
|
|---|---|
| RECOMMENDED |
RATING |
| Persons with current HCV infection should receive education and interventions aimed at reducing liver disease progression and preventing HCV transmission. | IIa, B |
| Abstinence from alcohol and, when appropriate, interventions to facilitate cessation of alcohol consumption should be advised for all persons with HCV infection. | IIa, B |
| Evaluation for other conditions that may accelerate liver fibrosis, including hepatitis B and HIV infections, is recommended for all persons with active HCV infection. | IIb, B |
| Evaluation for advanced hepatic fibrosis using noninvasive tests (serum panels, elastography) or liver biopsy, if required, is recommended for all persons with HCV infection to facilitate an appropriate decision regarding HCV treatment strategy, and to determine the need for initiating additional measures for cirrhosis management (eg, hepatocellular carcinoma screening) (see Monitoring section). | I, A |
| Vaccination against hepatitis A and hepatitis B is recommended for all susceptible persons with HCV infection. | IIa, C |
| Vaccination against pneumococcal infection is recommended for all patients with cirrhosis. | IIa, C |
| All persons with HCV infection should be provided education about how to prevent HCV transmission to others. | I, C |
In addition to receiving antiviral therapy, HCV-infected persons should be educated about how to prevent further liver damage. Most important is prevention of the potentially deleterious effects of alcohol. Numerous studies have found a strong association between excess alcohol use and the development or progression of liver fibrosis, and the development of hepatocellular carcinoma (Safdar, 2004); (Harris, 2001); (Bellentani, 1999); (Corrao, 1998); (Wiley, 1998); (Poynard, 1997); (Noda, 1996). Daily consumption of >50 g of alcohol has a high likelihood of worsening fibrosis. Some studies indicate that daily consumption of lesser amounts of alcohol also exerts a deleterious effect on the liver; these data, however, are controversial (Hagström, 2017); (Younossi, 2013b); (Westin, 2002). Persons who abuse alcohol and have alcohol dependence require treatment and consideration for referral to an addiction specialist.
Hepatitis B virus (HBV) and HIV coinfection have been associated with a poorer HCV prognosis in cohort studies (Puoti, 2017b); (Kruse, 2014); (Thein, 2008a); (Zarski, 1998). Because of overlapping risk factors for these infections and benefits associated with their identification and treatment, HCV-infected persons should be tested for HIV antibody and hepatitis B surface antigen (HBsAg), using standard screening assays (Moyer, 2013); (CDC, 2008). See USPSTF HIV screening recommendations and CDC hepatitis B screening recommendations for additional information. Persons who test positive for HBsAg require monitoring during HCV treatment because of HBV reactivation risk (Lee, 2018). Anti-HBV therapy may also be considered (see reactivation of HBV in the Monitoring section). Persons who test negative for HBsAg but positive for hepatitis B core antibodies (anti-HBc)—with or without hepatitis B surface antibodies (anti-HBs)—have resolved HBV infection in most cases; the risk of clinically significant HBV reactivation with HCV therapy is very low in this scenario and no further workup is required (Mücke, 2018). Patients should be counseled about how to reduce their risk of acquiring these infections; HBV vaccination is recommended when appropriate.
Assessment of Liver Disease Severity
The severity of liver disease associated with chronic HCV infection is a key factor in determining the initial and follow-up evaluation of patients. Noninvasive tests using serum biomarkers, elastography, or liver imaging allow for accurate diagnosis of cirrhosis in most individuals (see pretreatment workup in When and in Whom to Initiate HCV Therapy). Liver biopsy is rarely required but may be considered if other causes of liver disease are suspected.
Noninvasive methods frequently used to estimate liver disease severity include:
- Liver-directed physical exam (normal in most patients)
- Routine blood tests (eg, ALT, AST, albumin, bilirubin, international normalized ratio [INR], and CBC with platelet count)
- Serum fibrosis marker panels
- Transient elastography
- Liver imaging (eg, ultrasound or CT scan)
Simple calculations derived from routine blood tests—such as the serum AST-to-platelet ratio index (APRI) (Wai, 2003) and FIB-4 score (Sterling, 2006)—as well as assessment of liver surface nodularity and spleen size by liver ultrasound or other cross-sectional imaging modalities can help determine if patients with HCV have cirrhosis and associated portal hypertension. The presence of portal hypertension is associated with a greater likelihood of developing future hepatic complications in untreated patients (Chou, 2013); (Rockey, 2006). Elastography provides instant information regarding liver stiffness and can reliably distinguish patients with a high versus low likelihood of cirrhosis (Bonder, 2014); (Castera, 2012). A more detailed discussion regarding fibrosis assessment is found in the When and In Whom to Initiate Therapy section.
Persons with known or suspected bridging fibrosis and cirrhosis are at increased risk for developing complications of advanced liver disease and require frequent follow-up. They should also avoid hepatotoxic drugs, such as excessive acetaminophen (>2 g/d) and certain herbal supplements. Nephrotoxic drugs, such as nonsteroidal anti-inflammatory drugs, should also be avoided. Ongoing imaging surveillance for liver cancer and gastroesophageal varices is recommended for these patients (Fontana, 2010); (Sangiovanni, 2006). Persons with cirrhosis are more susceptible to invasive pneumococcal infection (Marrie, 2011) and should receive pneumococcal vaccination (CDC, 2012).
Exposure to infected blood is the primary mode of HCV transmission. HCV-infected persons must be informed of the precautions needed to avoid exposing others to infected blood. This is particularly important for PWID given that HCV transmission in this population primarily results from sharing needles and other contaminated drug injection equipment. Epidemics of acute HCV due to sexual transmission in HIV-infected men who have sex with men have also been described (Urbanus, 2009); (van de Laar, 2009); (Fierer, 2008). Table 1 outlines measures to avoid HCV transmission. HCV is not spread by sneezing, hugging, holding hands, coughing, or sharing eating utensils or drinking glasses, nor is it transmitted through food or water.
Table 1. Measures to Prevent HCV Transmission
| HCV-infected persons should be counseled to avoid sharing toothbrushes and dental or shaving equipment, and be cautioned to cover any bleeding wound to prevent the possibility of others coming into contact with their blood. |
Persons should be counseled about harm reduction related to illicit drug use, including offering medication for opioid use disorder, if appropriate, or referral to a substance use treatment program. Those who continue to inject drugs should be referred to local syringe services programs and counseled to (Platt, 2017):
|
| Persons with HCV infection should be advised not to donate blood and to discuss HCV serostatus prior to donation of body organs, other tissue, or semen. |
| Persons with HIV infection and those with multiple sexual partners or sexually transmitted infections should be encouraged to use barrier precautions to prevent sexual transmission. Other persons with HCV infection should be counseled that the risk of sexual transmission is low and may not warrant barrier protection. |
| Household surfaces and implements contaminated with visible blood from an HCV-infected person should be cleaned using a dilution of 1 part household bleach to 9 parts water. Gloves should be worn when cleaning up blood spills. |
Improved identification of active HCV infection and treatment advances will have limited impact on HCV-related morbidity and mortality without concomitant improvement in linkage to care. All patients with current HCV infection and a positive HCV-RNA test should be evaluated by a healthcare provider with expertise in assessment of liver disease severity and HCV treatment. Subspecialty care and consultation may be required for persons with HCV infection who have advanced fibrosis or cirrhosis (Metavir stage ≥F3), including possible referral for consideration of liver transplantation in those with evidence of hepatic decompensation.
Data do not support exclusion of HCV-infected persons from consideration for hepatitis C therapy based on alcohol intake or use of illicit drugs (see Identification and Management of HCV in People Who Inject Drugs). Some possible strategies to address HCV treatment barriers are listed in Table 2.
Table 2. Common Barriers to and Misconceptions Regarding HCV Treatment and Potential Strategies
Barrier |
Strategy |
|---|---|
|
Comorbid conditions (eg, substance use, psychiatric disorders, uncontrolled chronic medical conditions) |
|
|
Competing priorities and loss to follow-up |
|
|
Treatment adherence and adverse effects |
|
|
Lack of access to treatment (eg, out-of-pocket costs, high copays, lack of insurance, geographic distance, and/or lack of specialist availability) |
|
|
Lack of practitioner expertise |
|
Co-localization of HCV screening, evaluation, and treatment with other medical or social services (ie, integrated care) is a strategy that addresses several treatment barriers. Co-localization has already been applied to settings with high HCV prevalence (eg, correctional facilities, needle exchange programs, substance abuse treatment centers, and harm reduction programs), but this type of care is not uniformly available (Burton, 2019); (Harrison, 2019); (Morey, 2019); (Schulkind, 2019); (Bruggmann, 2013); (Islam, 2012); (Stein, 2012). A recent study demonstrated that integrated care—consisting of multidisciplinary care coordination and patient case management—increased the proportion of patients with HCV infection and psychiatric illness or substance use who begin antiviral therapy and achieve SVR without serious adverse events (Ho, 2015).
A strategy that addresses lack of access to specialists—a primary barrier to HCV care—is participation in models involving close collaboration between primary care practitioners and subspecialists (Beste, 2017b); (Rossaro, 2013); (Miller, 2012); (Arora, 2011). Such collaborations have used telemedicine and knowledge networks to overcome geographic distances to specialists (Rossaro, 2013); (Arora, 2011) or the availability of experienced providers in a methadone or correctional setting (Morey, 2019); (Talal, 2019). For example, project ECHO (Extension for Community Healthcare Outcomes) uses videoconferencing to enhance primary care practitioner capacity in rendering HCV care and treatment to New Mexico's large rural and underserved population (Arora, 2011). Through case-based learning and real-time feedback from a multidisciplinary team of specialists (gastroenterology, infectious disease, pharmacology, and psychiatry practitioners), project ECHO has expanded HCV treatment access in populations that might have otherwise remained untreated. The short duration of treatment and few serious adverse events associated with DAA therapy present an opportunity to expand the number of primary care providers engaged in HCV management and treatment. This expansion will support the goal of HCV elimination and overcome barriers associated with the need for subspecialty referrals. The ASCEND trial utilized a real-world cohort of patients at urban federally qualified health centers and found that HCV treatment administered by nonspecialist providers was as safe and effective as that provided by specialists (Kattakuzhy, 2017).
Additional strategies for enhancing linkage to and retention in care could be adapted from other fields, such as tuberculosis and HIV. For example, use of directly observed therapy has enhanced adherence to tuberculosis treatment, and use of case managers and patient navigators has reduced loss of follow-up in HIV care (Govindasamy, 2012). Recent HCV testing and care programs have identified the use of patient navigators or care coordinators as important interventions in overcoming challenges associated with linkage to and retention in care (Ford, 2018); (Coyle, 2015); (Trooskin, 2015). There are also data suggesting that financial incentives and peer navigation may be useful to support treatment adherence in patients with substance use disorders (Ward, 2019); (Wohl, 2017). Ongoing assessment of efficacy and comparative effectiveness of this and additional strategies is a crucial area of future research for patients with HCV infection. Replication and expansion of best practices and new models for linkage to HCV care will also be crucial to maximize the public health impact of newer treatment paradigms.
- Related References


