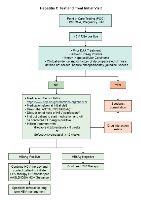
Simplified HCV Treatment Algorithm for Treatment-Naive Adults With Compensated Cirrhosis
Who Is NOT Eligible for Simplified Treatment (With Cirrhosis) |
|---|
|
Patients who have any of the following characteristics:
(see HCV guidance for treatment recommendations for these patients) |
Who Is Eligible for Simplified Treatment |
|---|
|
Adults with chronic hepatitis C (any genotype) who have compensated cirrhosis (Child-Pugh A) and have not previously received hepatitis C treatment Liver biopsy is not required. For the purpose of this guidance, a patient is presumed to have cirrhosis if they have a FIB-4 score >3.25 or any of the following findings from a previously performed test.
|
- Calculate FIB-4 score.
- Calculate CTP score: Patients with a CTP score ≥7 (ie, CTP B or C) have decompensated cirrhosis and this simplified treatment approach is not recommended.
- Ultrasound of the liver (conducted within the prior 6 months): Evaluate to exclude HCC and subclinical ascites.
- Medication reconciliation: Record current medications, including over-the-counter drugs and herbal/dietary supplements.
-
Potential drug-drug interaction assessment: Drug-drug interactions can be assessed using the AASLD/IDSA guidance or the University of Liverpool drug interaction checker.
- Drug-drug interactions are particularly important in HIV co-infection
- In those with HIV, the simplified treatment approach should not be used in those on TDF containing regimens with eGFR <60 ml/min because of the need of additional monitoring.
- Education: Educate the patient about proper administration of medications, adherence, and prevention of reinfection.
-
Pretreatment laboratory testing:
-
Within 3 months of initiating treatment:
- Complete blood count (CBC)
- International normalized ratio (INR)
- Hepatic function panel (ie, albumin, total and direct bilirubin, alanine aminotransferase [ALT], aspartate aminotransferase [AST])
- Calculated glomerular filtration rate (eGFR)
-
Any time prior to starting antiviral therapy:
- Quantitative HCV RNA (HCV viral load)
- HIV antigen/antibody test
- Hepatitis B surface antigen
- HCV genotype (if treating with sofosbuvir/velpatasvir)
-
Before initiating antiviral therapy:
- Serum pregnancy testing and counseling about pregnancy risks of HCV medication should be offered to women of childbearing age.
-
Within 3 months of initiating treatment:
-
Genotype 1-6:
Glecaprevir (300 mg) / pibrentasvir (120 mg) to be taken with food for a duration of 8 weeks
-
Genotype 1, 2, 4, 5, or 6
Sofosbuvir (400 mg) / velpatasvir (100 mg) for a duration of 12 weeks
NOTE: Patients with genotype 3 require baseline NS5A resistance-associated substitution (RAS) testing. Those without Y93H can be treated with 12 weeks of sofosbuvir/velpatasvir. If Y93H is present, see HCV guidance for treatment recommendations.
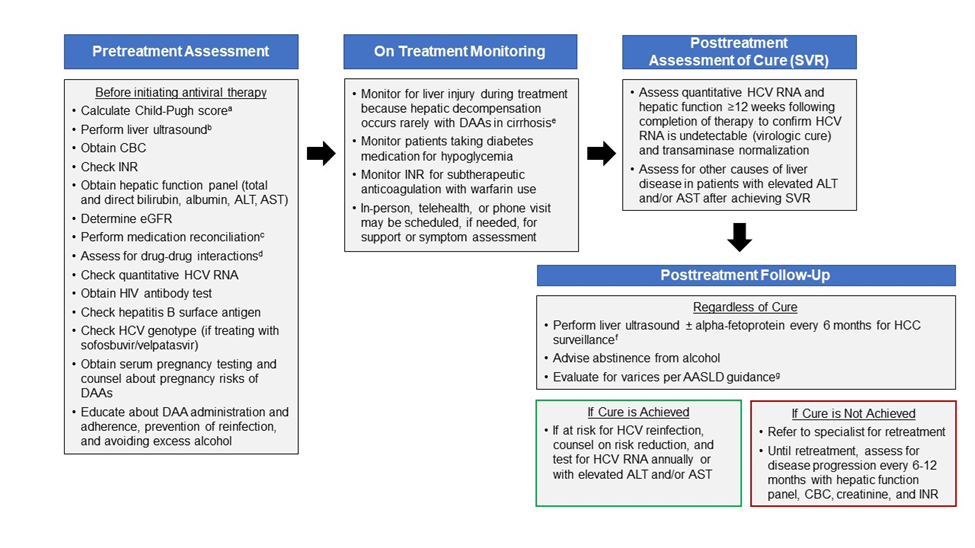
- Providers may order blood tests to monitor for liver injury during treatment because hepatic decompensation (eg, jaundice, etc) occurs rarely among patients with cirrhosis receiving HCV antiviral treatment.
- Patients should see a specialist if they develop worsening liver blood tests (eg, bilirubin, AST, ALT, etc); jaundice, ascites, or encephalopathy; or new liver-related symptoms.
- Inform patients taking diabetes medication of the potential for symptomatic hypoglycemia. Monitoring for hypoglycemia is recommended.
- Inform patients taking warfarin of the potential for changes in their anticoagulation status. Monitoring INR for subtherapeutic anticoagulation is recommended.
- An in-person or telehealth/phone visit may be scheduled, if needed, for patient support, assessment of symptoms, and/or new medications.
- Assessment of quantitative HCV RNA and a hepatic function panel are recommended 12 weeks or later following completion of therapy to confirm HCV RNA is undetectable (virologic cure) and transaminase normalization.
- Assessment for other causes of liver disease is recommended for patients with elevated transaminase levels after achieving SVR.
- Ultrasound surveillance for HCC (with or without alpha-fetoprotein testing) every 6 months is recommended for patients with cirrhosis in accordance with AASLD guidance.
- Upper endoscopic surveillance for esophageal varices is recommended in accordance with AASLD guidance on portal hypertensive bleeding in cirrhosis.
- Patients with ongoing risk for HCV infection (eg, intravenous drug use or MSM engaging in unprotected sex) should be counseled about risk reduction, and tested for HCV RNA annually and whenever they develop elevated ALT, AST, or bilirubin.
- Patients should abstain from alcohol to avoid progression of liver disease.
- Patients in whom initial HCV treatment fails to achieve cure (SVR) should be evaluated for retreatment by a specialist, in accordance with AASLD/IDSA guidance.
- Ultrasound surveillance for hepatocellular carcinoma (with or without alpha-fetoprotein testing) every 6 months is recommended for patients with cirrhosis, in accordance with AASLD guidance.
- Assessment for disease progression every 6 to 12 months with a hepatic function panel, CBC, creatinine, and INR is recommended.
- Patients should abstain from alcohol to avoid progression of liver disease.
*More detailed descriptions of the patient evaluation process and antivirals used for HCV treatment can be found here.
|
a Child-Pugh score based on presence of ascites, hepatic encephalopathy, total bilirubin >2.0 mg/dL, albumin ≤3.5 g/dL, or INR ≥1.7. Patients with a Child-Pugh score ≥7 (ie, Child-Pugh B or C) have decompensated cirrhosis; this simplified treatment approach is not recommended for patients with decompensated cirrhosis. b Obtain liver ultrasound within 6 months prior to initiating antiviral treatment to exclude hepatocellular carcinoma and subclinical ascites. This simplified treatment approach is not recommended for patients with hepatocellular carcinoma and/or decompensated cirrhosis. c Medication reconciliation should record currently prescribed medications, over-the-counter drugs, and herbal/dietary supplements. d Drug-drug interaction assessment should be performed using the table in the monitoring section of the HCV guidance website or the University of Liverpool drug interaction checker. e Development of jaundice, ascites, spontaneous bacterial peritonitis, variceal hemorrhage, or hepatic encephalopathy may suggest hepatic decompensation. Patients should be referred to a specialist if they develop worsening liver blood tests (eg, total bilirubin, AST, ALT, INR), jaundice, ascites, encephalopathy, or new liver-related symptoms). f Ultrasound surveillance for hepatocellular carcinoma (with or without alpha-fetoprotein testing) every 6 months is recommended for patients with cirrhosis, in accordance with AASLD guidance. g See AASLD guidance for recommendations regarding the evaluation and management of varices. |
Download the New Hepatitis C Point of Care Test and Treat Algorithm
Other Downloads:
 Simplified HCV Treatment* for Treatment-Naive Patients
Simplified HCV Treatment* for Treatment-Naive Patients
Without Cirrhosis - Click here to download the PDF, or read more.
With Compensated Cirrhosis - Click here to download the PDF, or read more.